THIS STUDY IS CLOSED TO ENROLLMENT.
This research aims to measure and track behavioral characteristics of depression and use these measures to predict brain activity, as measured by MRI scans. If you live in the Los Angeles area, you may be eligible to participate.
You must be willing and eligible to have an MRI scan and provide a blood sample in order to participate. If you are taking antidepressants, in order to be eligible your medication and dose must have been stable for the past 2 weeks. Individuals who have metal implants, braces, are currently pregnant, or who are claustrophobic are unable to participate.
You will be asked if you are willing to participate in a follow-up study, which involves low-intensity focused ultrasound pulsation (LIFUP) and an additional month of assessments, if eligible. This is optional, you are not required to participate in any study.
To participate you must have an iPhone 8 or later, operating on iOS 15 or newer, and with access to data plan and Wifi. Participants in this study must download an app to their personal iPhone. This app is not available to androids or iPads, only iPhone 8 or later models can be used.
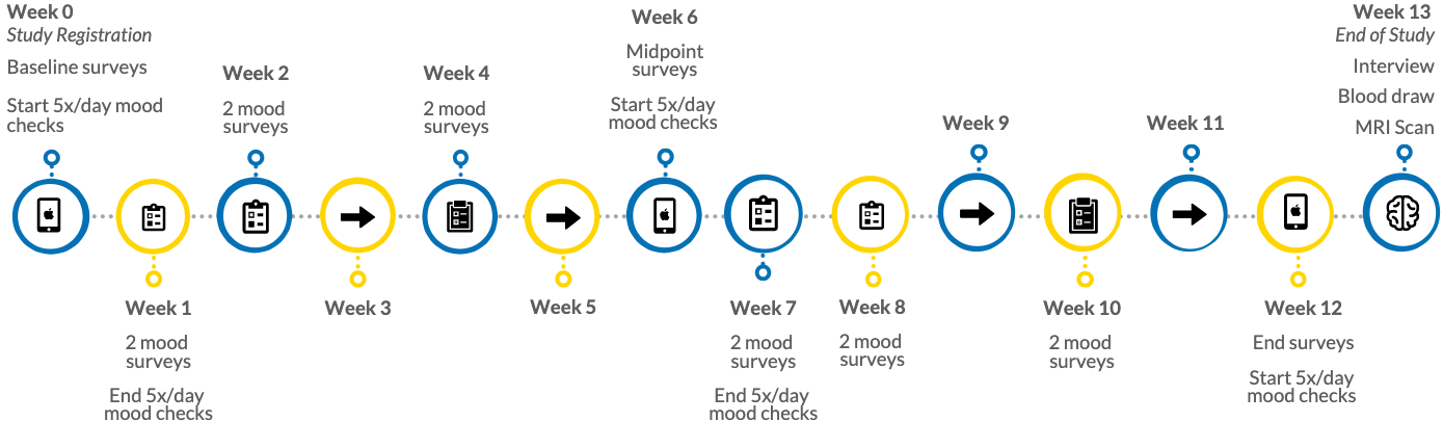
Research participation lasts approximately 3.5 months. At three time points (baseline, week 6, and week 12), you will be asked to complete a set of online self-report surveys, and complete one week of mood surveys (respond to 5 daily prompts (1 minute each) for 8-days straight). You will be asked to additionally complete brief symptom assessments 1-2 weeks and wear an Apple Watch (which will be provided to you) for the duration of the study.
After 12 weeks of data collection, you will be asked to visit our research offices at UCLA and complete an MRI scan, during which you will remain still and relaxed with your eyes open, and at times complete computer tasks. During this visit you will also be asked to provide a blood sample, and participate in a brief, recorded interview. The recorded interview will last about 10 minutes, and we will ask you to describe positive and negative experiences you had in your past week.
Willingness to provide a blood sample and an audio/video recorded interview is required for study participation. Based on your responses to surveys and your study participation, you may be asked to participate in a second, optional study involving low-intensity focused ultrasound pulsation and an additional 4 weeks of data collection.
Study participation is expected to require a total of about 8.75 hours over approximately 3.5 months. You can earn up to $374 for completing all study assessments. If you complete all of the assessments and the MRI scan, you will be able to keep the Apple Watch.
Participation is entirely voluntary. This study is not to provide treatment.
In order to determine if you are eligible, you will be asked to answer a series of questions about yourself and answer questions about current symptoms of depression that you may be experiencing. If you are eligible based on your responses, you will be routed to the consent form, which you will have an opportunity to read before deciding to enroll. You will also be asked to schedule a follow-up phone screening with study staff to confirm eligibility.
Participants in this study are asked to undergo a 2 hour long MRI scan, during which you must lie very still, with the upper half of your body and head in a confined space, while a piece of equipment is covering much of your head. If this sounds extremely uncomfortable or claustrophobic to you, we do not recommend you enroll. If you are unsure, please continue through these pages to enroll, and you can then speak with staff about the MRI scan procedures. If you are unsure after speaking with our staff, you could be scheduled to come to UCLA for an imitation scan to see if you would be comfortable, before choosing to enroll.
If you are not eligible to enroll at this time based on your responses, you will be asked whether you would like to be contacted at a later date if enrollment opens to you.
This screening may take 20-30 minutes to complete. If any of these webpages remain idle, you will be logged out. You can re-enter your email and phone number, and verify your number via text message, to resume.
For a list of Emergency and Outpatient Resources, click here.
To continue, please select Next.